BARDA partners with Hoxworth Blood Center to develop and validate an improved method to evaluate next-generation blood products
To further prioritize the advanced development of next-generation blood products for use, BARDA is partnering with Hoxworth Blood Center, University of Cincinnati, to develop an improved alternative method to assess blood products in regulatory studies.
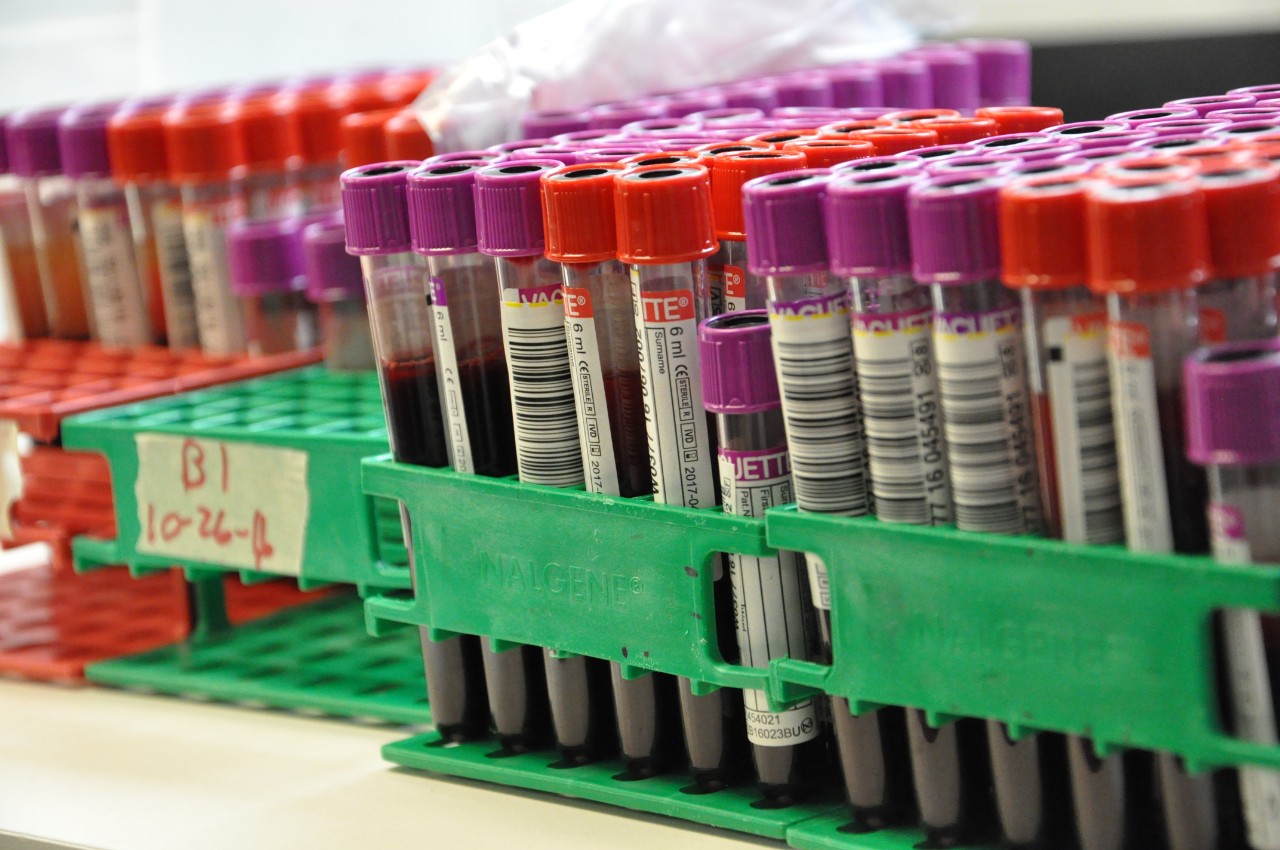
Hoxworth Blood Center will conduct pre-clinical and clinical trials to compare Biotinylation to Chromium-51 to evaluate the safety and efficacy of using a non-radioactive alternative labeling method to assess blood products. If biotin is found to be a more effective labeling alternative, it could result in many improvements of safety and availability of blood products in radiological or nuclear mass casualty incidents (MCI).
Biotin (vitamin B7) is an essential nutrient that helps the human body metabolize fatty acids, glucose, and amino acids and is naturally present in food and is available as a dietary supplement. Since it is more widely found, it could help improve the amount of blood products available for patients that need it. Additionally, the U.S. has only one producer of Chromium-51 which could result in less product availability and potential shortages.
Cr-51 is a synthetic radioactive isotope of chromium used to label RBCs to facilitate measurement of circulating RBC mass, volume, and survival time. The use of Cr-51 exposes research subjects and laboratory technicians to radiation and limits the number of laboratories permitted to perform testing due to regulation requirements, such as needing to obtain a special license and may be subject to state or U.S. Nuclear Regulatory Commission guidelines for possessing/using radioactive materials. Additionally, more laboratories could evaluate blood products and novel blood products candidates, including those for use in transfusion in the context of public health emergencies and routine medical treatment and would be a safer alternative to using Cr-51 because it is not radioactive.
Under the agreement, BARDA will provide funding and expertise for a clinical trial to validate biotin, a naturally occurring vitamin, as a safe and effective option to replace chromium 51 (Cr-51) as the U.S. Food and Drug Administration’s (FDA’s) current “gold standard” labeling method for measurement of circulating red blood cells (RBCs). BARDA invests in next generation blood product candidates that address hematopoietic injuries and build blood system capacity, including those that have long shelf-lives, are stable at higher temperatures, and can be administered in the field or enroute to a hospital. These investments rely on enabling technologies, such as safer labeling methods that enable evaluation of circulating RBCs, to support regulatory approval.
This award contributes to objectives set in BARDA’s 2022-2026 Strategic Plan, which emphasizes the importance of supporting the development of enabling technologies that can facilitate the regulatory process.
This award is one component of the BARDA’s CBRN Division medical countermeasure portfolio, visit the CBRN Portfolio to learn more.
About Hoxworth:
Hoxworth Blood Center, University of Cincinnati, was founded in 1938 and serves more than 30 hospitals in 18 counties in Southwestern Ohio, Northern Kentucky, and Southeastern Indiana. Annually, Hoxworth collects more than 100,000 units of blood from local donors to help save the lives of patients in area hospitals. Hoxworth Blood Center: Saving Lives Close to Home.
Related Stories
Sugar overload killing hearts
November 10, 2025
Two in five people will be told they have diabetes during their lifetime. And people who have diabetes are twice as likely to develop heart disease. One of the deadliest dangers? Diabetic cardiomyopathy. But groundbreaking University of Cincinnati research hopes to stop and even reverse the damage before it’s too late.
App turns smartwatch into detector of structural heart disease
November 10, 2025
An app that uses an AI model to read a single-lead ECG from a smartwatch can detect structural heart disease, researchers reported at the 2025 Scientific Sessions of the American Heart Association. Although the technology requires further validation, researchers said it could help improve the identification of patients with heart failure, valvular conditions and left ventricular hypertrophy before they become symptomatic, which could improve the prognosis for people with these conditions.
Combination immunotherapy helps overcome melanoma treatment resistance
November 10, 2025
MSN highlighted research led by the University of Cincinnati Cancer Center's Trisha Wise-Draper showing a combination of immunotherapy medications can activate a robust immune response and help overcome treatment resistance in patients with refractory melanoma.
